The reason why defoamer can effectively inhibit and eliminate foam is that there are complex physical and chemical principles behind it. In this paper, the mechanism of defoaming powder will be deeply analyzed from the formation mechanism of foam.
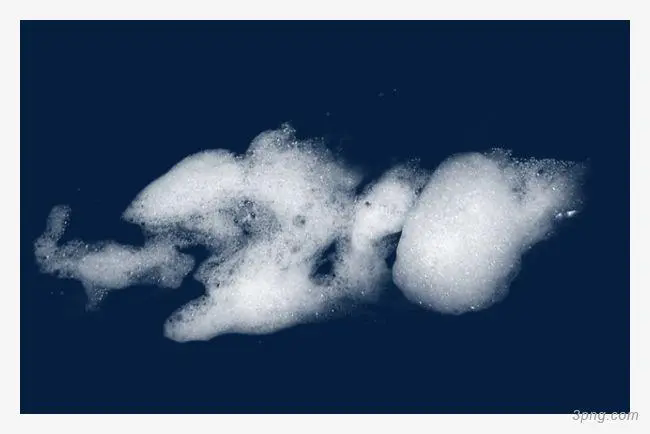
Foam is a dispersion system formed by gas dispersion in liquid or solid, and its stability depends on the surface tension of the bubble film. When there is surfactant in the liquid, the surface tension of the bubble film decreases, and the foam is more stable.
Surface tension destruction: Defoamer molecules can rapidly diffuse to the surface of foam, reduce its surface tension, and make foam film fragile and easy to break.Spreading and penetration: some defoamer particles can penetrate the foam film and enter into the bubble. The bubble will shrink and break rapidly through the internal pressure difference.Inhibition of regeneration: the effective ingredients in defoaming powder can also form a film on the liquid surface, blocking the formation of new foam, so as to achieve long-term defoaming.
The defoaming effect is influenced by various factors such as the type, dosage, addition method, system temperature, pH value, and stirring intensity of defoaming powder. The correct selection and use of defoaming powder is the key to ensuring the effectiveness of defoaming.

 English
English
 Chinese
Chinese Vietnamese
Vietnamese
 HOME
HOME
 PRODUCT
PRODUCT
 NEWS
NEWS
 CONTACT
CONTACT


